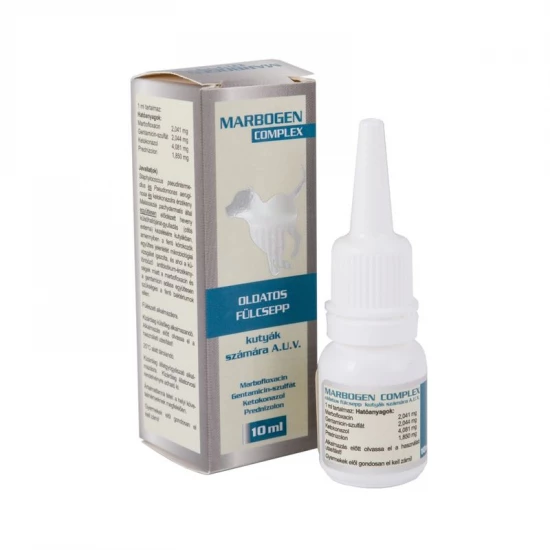
Hatóanyag – 1 ml tartalmaz:
Marbofloxacin: 2,041 mg
Gentamicin-szulfát 2,044 mg
Ketokonazol 4, 081 mg
Prednizolon 1, 850 mg
Gyógyszerforma: oldatos fülcsepp
Célállat faj: kutya.
Terápiás javallat: kutyák Staphylococcus pseudointermedius, Pseudomonas aeruginosa és ketokonazolra érzékeny Malassezia pachydermatis okozta heveny külső hallójárat-gyulladásának (otitis externa) kezelésére.
Adagolás: öt cseppet (0,1 ml) kell naponta kétszer a hallójáratba csepegtetni, 14 napon keresztül.
Élelmezés-egészségügyi várakozási idő: Nem értelmezhető.
Kiadhatóság: állatorvosi rendelvényre
Csomagolás: 10 ml cseppentős műanyag flakonban.
1. NAME OF THE VETERINARY MEDICINAL PRODUCT
MARBOGEN COMPLEX ear drops solution for dogs
GENAURIS ear drops solution for dogs
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml contains:
Active substances:
Marbofloxacin 2.041 mg
Gentamicin sulfate 2.044 mg
Ketoconazole 4.081 mg
Prednisolone 1.850 mg
Excipients:
For a full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Ear drops, solution
Yellowish, clear to almost clear solution
4. CLINICAL PARTICULARS
4.1 Target species
Dogs
4.5 Special precautions for use
Special precautions for use in animals
HU/V/0123/001/DC - SPC, labelling, PL 2 / 14 Day 210 - 25.05.2016
Use of the veterinary medicinal product should be based on identification of infecting organisms and susceptibility testing, taking into account official and local antimicrobial policies. Care should be taken that diagnostic procedures are not neglected due to the wide spectra of the antimicrobial components.
Diagnostic procedure should include physical examination, cytology examination, and taking swab samples. Samples should be cultured, pathogen microbes and their resistance pattern should be determined.
Heavy reliance on a single class of antibiotic may result in the induction of resistance in a bacterial population. It is prudent to reserve fluoroquinolones for the treatment of clinical conditions, which have responded poorly or are expected to respond poorly to other classes of antibiotics.
Prolonged and intensive use of topical corticosteroids preparation is known to trigger local and systemic effects, including suppression of adrenal function, thinning of the epidermis and delayed healing.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
People with known hypersensitivity to any of the ingredients should avoid contact with the product.
Personal protective equipment consisting of impermeable gloves should be worn when handling the product.
Do not eat, drink or smoke when administering the product.
In case of skin exposition clean the contaminated area with a water-soap solution.
In case eye contact, wash immediately with abundant water.
Seek medical advice if signs of cutaneous erythema, exanthema, or persistent ocular irritation appear after exposure. Swelling of face, lips and eyes, or respiratory difficulties are more serious signs that need urgent medical action.
6.5 Nature and composition of immediate packaging
10 ml white LDPE bottle assembled with white LDPE dropper and closed with white HDPE cap.